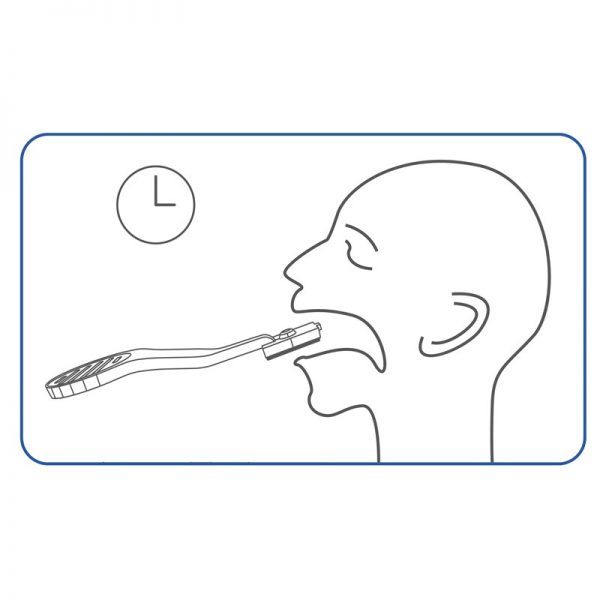
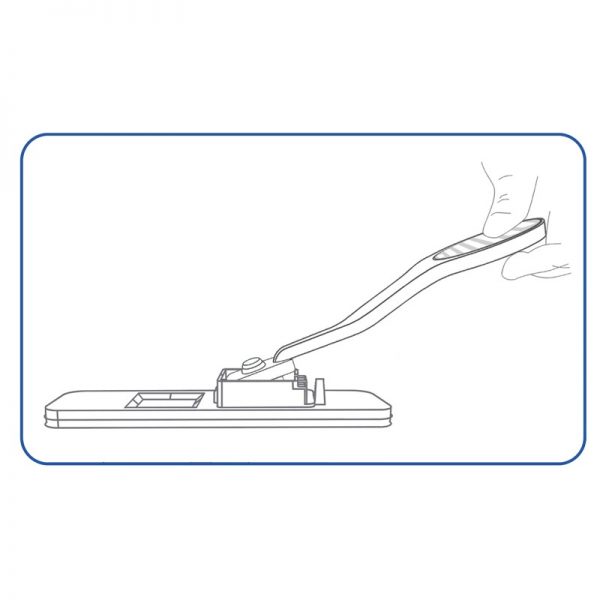
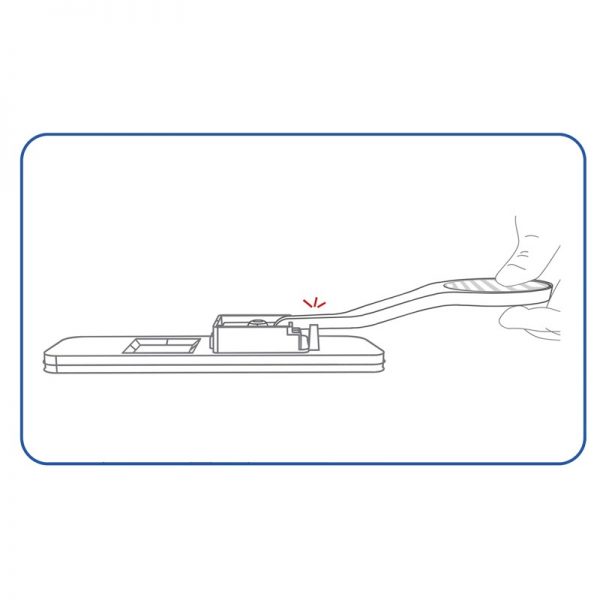
professional ulti med Corona saliva test
- Article ID
- 012G521
- manufacturer
- ulti med Products (Deutschland) GmbH
- Handling time
- / /
Product description
Rapid test for the qualitative detection of COVID-19 Antigens in human saliva.
For professional in vitro diagnostic use only.
The ulti med COVID-19 Antigen Saliva Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from 2019-nCoV in saliva specimens directly collected from individuals who are suspected of COVID-19 by their healthcare provider within the first 7 days of symptom onset. Results are for the identification of 2019-nCoV nucleocapsid protein antigen.
PRINCIPLE
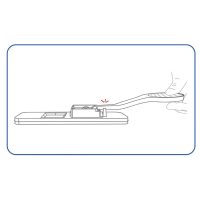
The ulti med COVID-19 Antigen Saliva Test uses double-antibody sandwich to detect the antigen of novel coronavirus (2019-nCoV) in saliva samples. During detection, the gold labeled anti-2019-nCoV monoclonal antibody in the labeling pad binds to the 2019-nCoV antigen in the sample to form a complex, and the reaction complex moves forward along the nitrocellulose membrane under the action of chromatography. It is captured by the anti-2019-nCoV monoclonal antibody pre-coated on the detection zone (T) on the nitrocellulose membrane, and finally a red color reaction line is formed in the T zone. If the sample does not contain 2019-nCoV antigen, a red color reaction line cannot be formed in the T zone. If the sample does not contain 2019-nCoV antigens, a red reaction line cannot be formed in the T zone. Regardless of whether the sample to be tested contains 2019-nCoV antigens, a red reaction line will always form in the quality control area (C).
Advantages
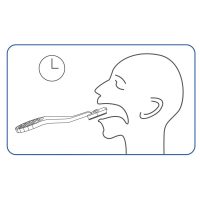
- Painless sampling due to saliva collection in the mouth
- Higher acceptance by test subjects
- Higher willingness for prophylactic, frequent testing
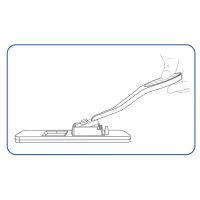
- Simplest test performance without handling, such as diluting and pipetting, with a possibly infectious sample
- Also suitable for minors and bedridden people, as well as for people with allergies or under anesthesia
- Rapid result after 10 minutes
BfArm no. AT075/21
PEI tested